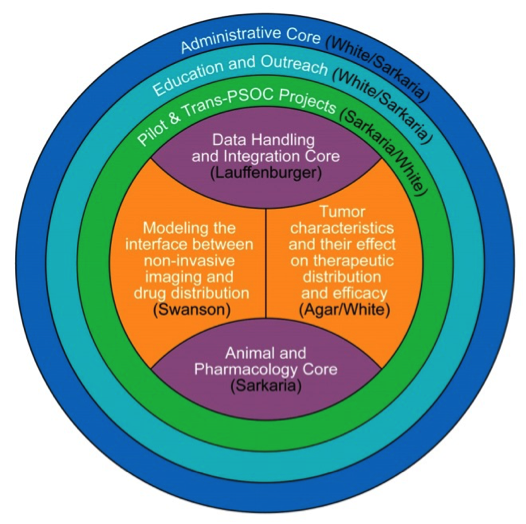
 A central goal of the MIT/Mayo PS-OC is to model the physical and physico-chemical factors that govern drug distribution into brain tumors. Integrated with precision medicine strategies to define key molecular vulnerabilities based on genomic analyses, our multi-scale modeling analysis of drug distribution could be instrumental in identifying corresponding targeted therapeutics with the highest likelihood of optimal drug distribution across the entire tumor cell population in an individual brain tumor. Given the complex and daunting nature of this critically important clinical task, we have assembled a team of physical scientists, engineers, and oncologists with a wide range of expertise spanning cutting-edge imaging, systems biology, computational modeling, and neurooncology. This team is organized into two highly integrated Research Projects and two shared Resource Cores. Administration of these activities, along with future Pilot Projects and trans-PS-OC projects will be accomplished by the Administration Core, while the Education and Outreach Core will ensure the communication and extension of these activities to the larger physical oncology community.
A central goal of the MIT/Mayo PS-OC is to model the physical and physico-chemical factors that govern drug distribution into brain tumors. Integrated with precision medicine strategies to define key molecular vulnerabilities based on genomic analyses, our multi-scale modeling analysis of drug distribution could be instrumental in identifying corresponding targeted therapeutics with the highest likelihood of optimal drug distribution across the entire tumor cell population in an individual brain tumor. Given the complex and daunting nature of this critically important clinical task, we have assembled a team of physical scientists, engineers, and oncologists with a wide range of expertise spanning cutting-edge imaging, systems biology, computational modeling, and neurooncology. This team is organized into two highly integrated Research Projects and two shared Resource Cores. Administration of these activities, along with future Pilot Projects and trans-PS-OC projects will be accomplished by the Administration Core, while the Education and Outreach Core will ensure the communication and extension of these activities to the larger physical oncology community.
Project 1 - Modeling the interface between non-invasive imaging and drug distribution (Swanson, Hu, Ma, Parney) is led by Dr. Kristin Swanson, an expert on mathematical modeling of brain tumor imaging with regards to tumor distribution and patterns of tumor growth. This project aims to merge classic pharmacokinetics, MR imaging and 3-dimensional (3D) drug delivery measurements in animal models and in human tumors to define critical physical parameters that govern macroscopic drug distribution at the level of tumor tissue.
Project 2 – Tumor Characteristics and their effect on therapeutic distribution and efficacy (Agar, White, Tran) is co-led by Drs. Agar and White, experts in the application of mass spectrometry to evaluate 3D drug distribution and the impact of drug therapy on signaling network perturbation, respectively. This second project will focus on identifying key determinants that drive the cellular and sub-cellular distribution of drug within a tumor tissue and defining the effects of drug distribution on signaling and transcriptional networks and the corresponding cellular response.
The Administrative Core (Sarkaria and White) and the two Shared Resource Cores provide the mechanisms that support both projects and ensure robust integration of the entire project. In close collaboration with the Project teams, the Animal and Pharmacology Core (Sarkaria and Elmquist) will organize and execute the animal tumor model studies involving therapy evaluation, pharmacokinetics, drug distribution, and imaging studies. The Animal and Pharmacology Core personnel will distribute both animal and human bio-specimens to the relevant scientific personnel. The multi-scale data obtained from each bio-specimen will be handed off from relevant laboratories to the Data Handling and Integration Core (Lauffenburger, Wittrup, Agar, and Swanson). This Core will organize the multiple imaging and systems biology data sets (MRI, Stimulated Raman Scattering (SRS) imaging, MALDI-MSI, immunohistochemistry (IHC), phosphoproteomics, proximity ligation assays (PLA), RNASeq) collected for each bio-specimen, extract data from these images and integrate these data sets to develop multi-scale models of drug delivery in close collaboration with the personnel from Projects 1 and 2.